FDA CE Medical Alginate Dressing for Wound Care/ Venous and Arterial Leg Ulcer/ Diabetic Ulcer/ Donor Sites
Description:LUOFUCON Silver Alginate Dressing is a sterilized wound dressing contain alginate fibers derived from seawee;
Basic Info.
| Model NO. | LW-D-005 |
| Logo Printing | Both Ok |
| Donor Sites | Lacerations |
| Post Surgical Wounds | Alginate Rope |
| Venous/Arterial Leg Ulcer | Diabetic Ulcer |
| FDA Listing | CE |
| Transport Package | PE Film Packaging |
| Specification | 10 pcs per package and customization |
| Trademark | LUOFUCON |
| Origin | Huizhou, Guangdong, China |
| HS Code | 3005901000 |
| Production Capacity | 50, 000, 000 PCS/Year |
Product Description

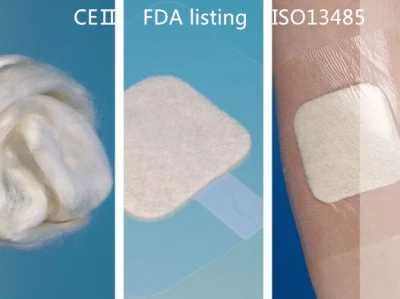
Description:LUOFUCON Silver Alginate Dressing is a sterilized wound dressing contain alginate fibers derived from seaweed. This wound dressing can absorb wound exudates and form a gel-like covering over the wound. The gel maintains a moist environment to accelerate wound healing. Meanwhile, the alginate gel will not adhere to wound during dressing changes, which avoids second damage.Features:CE CertificateHighly absorbent.Maintain moist environment.Easy application and removal.Indications:Alginate dressing can absorb up to 12 times its weight of fluid. It can be used in moderately to heavily exuding wound.Venous/arterial leg ulcer.Diabetic ulcer, pressure ulcer.Donor sites, abrasions, lacerations and post-surgical wound.
Other Premium Products of US:
About US:

You may also like
Send inquiry
Send now